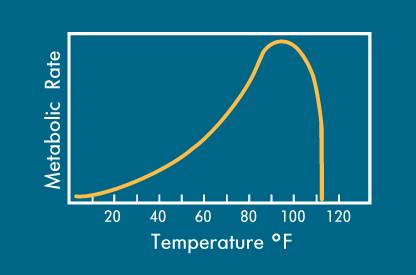
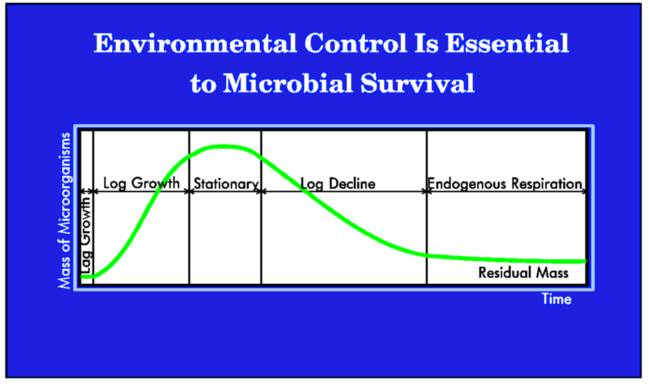
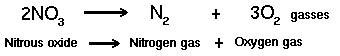Bacterial MetabolismThe biochemical reactions in living cells, which allow them to assimilate food to provide energy for their growth and reproduction, are termed metabolisms. During aerobic reaction, bacteria oxidize one-third of the organic matter present, yielding enough energy to synthesize the remaining organics into additional living cells, carbon dioxide and water. When obtaining their nutrients in an anaerobic reaction, the by-products are organic acids, aldehydes, ketones, and alcohols. The rate at which metabolism occurs is limited by the amount of available nutrients, the specific strains of bacteria present and their efficiency. Temperature and pH levels also limits bacterial growth rates. As metabolism continues, the number of microbes increases and the food supply is consumed at a faster rate. More efficient bacteria degrade organic wastes faster. 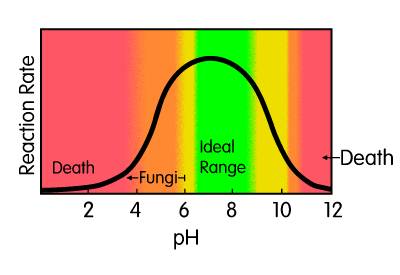 The initial growth phase is called log growth, since the microbes metabolize at a rate which doubles their number (cell division) and mass over a given time. Log growth rates vary from strain to strain.
At some point, the diminishing food supply begins to limit the growth rate. In this declining growth phase there are too many microorganisms and not enough food to sustain them. The endogenous respiration phase begins when the food can no longer support continued reproductive growth. Some cells remain viable or change to a dormant state (spore formation), while reproduction rates decrease. Other cells will self-destruct (autolysis), forming an additional nutrient source for the remaining living cells. Massive cell death occurs when all sources of nutrients are removed or toxins rise above a tolerable level. Bacteria (and Cyanobacteria a.k.a. blue-green algae and Araceae) are from the Monera kingdom, with the scientific designation of Schizophyta, neither plant nor animal. Bacteria exhibit a greater variety of mechanisms for obtaining energy than any other species of organisms. Bacteria are classified on the basis of their requirements for free atmospheric oxygen. Those that require oxygen are obligate aerobes, such as most strains of Pseudomonas fluorescens. Those bacterial strains that cannot live in the presence of oxygen are obligate anaerobes, such as methanogens. Those bacterial strains that can thrive with or without oxygen are facultative anaerobes, such as various bacilli and Pseudomonas aeruginosa. Strains that require low concentrations of oxygen, but cannot survive with either high oxygen or without oxygen, are microaerophiles, such as Enterobacter aerogenes. Bacteria are also classed by their temperature preferences: psychrophiles (5° to 20°C), mesophiles (20° to 45°C), thermophiles (45° to 80°C) and extreme thermophiles (>80°C). Please note that various sources use different parameters for these temperature categories, so it is important to verify that all persons in a conversation are dealing with common definitions. Heterotrophic bacteria (microorganisms which use organic matter for energy & growth) and autotrophic bacteria (microorganisms which obtain energy from inorganic materials using CO2 as their sole carbon source) utilize the organic excretions of the animals as energy sources. Ideally, aerobic bacteria will liquefy and digest organic nutrients and alleviate much of the toxic buildup from decomposition. When the desired natural bacterial populations are insufficient, inoculation with the correct synergistic blend of active bacteria will enhance the decomposition process. Various Alken Clear-Flo® formulas capitalize on several strains of facultatively anaerobic gram-negative rods that utilize glucose oxidatively. These strains possess the capacity to survive in conditions that few other organisms can tolerate, producing a slime layer that is not only resistant to phagocytosis, but provides shelter for other symbiotic strains in many Alken Clear-Flo® formulas. Alken-Murray also employs two strains which are part of the normal flora in the digestive tract of animals, which degrade fats and ferulate (4-hydroxy-3-methoxy-cinnamic acid, sodium salt; a common feed preservative) and six strains, selected for their general waste and urine degrading capacity. Certain of these strains produce antibiotics that inhibit the growth of certain pathogenic strains. Most facultative and aerobic organisms contain a high concentration of an enzyme called superoxide dismutase. Those that do not possess a high concentration of superoxide dismutase, possess catalase or peroxidase. The superoxide dismutase enzyme converts the superoxide anion into ground-state oxygen and hydrogen peroxide, thus ridding the cell of destructive superoxide anions: The hydrogen peroxide generated in this reaction is an oxidizing agent, but it does not damage the cell as much as the superoxide anion and tends to diffuse out of the cell. Many organisms possess catalase or peroxidase or both to eliminate the H2O2. The catalase enzyme uses H2O2 as an oxidant (an electron acceptor) and a reductant (an electron donor) to convert peroxide into water and ground-state oxygen: Peroxidase uses a reductant other than H2O2: Denitrification is an anaerobic reductive mechanism in which nitrate is reduced to nitrogen gas. Facultative anaerobes are the only bacteria capable of performing this activity
.
In the case above, nitrate is converted to nitrogen gas, by the process of "true denitrification" or DISSIMILATORY NITRATE REDUCTION, in which nitrate is replacing oxygen as the electron acceptor in the biochemistry of the cell. Only facultative anaerobic bacteria carry out this process. The change to using nitrate instead of oxygen, when oxygen is used up or is missing from the environment, is known as NITRATE RESPIRATION. On the other hand, when nitrate is converted to a glutamate, the process is called NITRATE ASSIMILATION or ASSIMILATORY NITRATE REDUCTION. Denitrification is one of the major causes of loss of N from soils. Other losses do occur as a result of volatilization, non-enzymatic reactions of nitrous acid with ammonium salts or amino acids, and chemical decomposition of nitrites under acid conditions to give nitrogen oxides. Most waste situations do NOT maintain a steady
organic load. When the load decreases, the cell growth declines. When the
load again increases, the remaining viable cells and dormant cells (spores)
are neither sufficiently young, nor are they present in sufficient concentration
to be effective.
 |