Phthalates, prominently used in many industries, including plastics, belong to a family of chemical compounds which are based on a benzene ring, to which is attached a pair of carbonyl groups in consecutive positions on the benzene ring. Commentary & examples: A benzene ring consists of a backbone of 6 carbons and 6 hydrogens. This structure can be represented as if it was alternating between 1,3,5-cyclohexatriene (a) and 2,4,6-cyclohexatriene (b), but the truth is that the extra electrons are not localized over the double bonds between the carbons. Single hydrogen atoms would also be associated with each carbon represented in the drawing. In reality, the electrons of the benzene ring are gathered into doughnut shaped clouds above and below the ring. Therefore, a simplified representation was adopted by chemists to represent the six carbons and their implicit six hydrogens, as a six sided figure (each point represents a carbon atom), and a circle in its center (the doughnut shaped electron cloud) (c) |
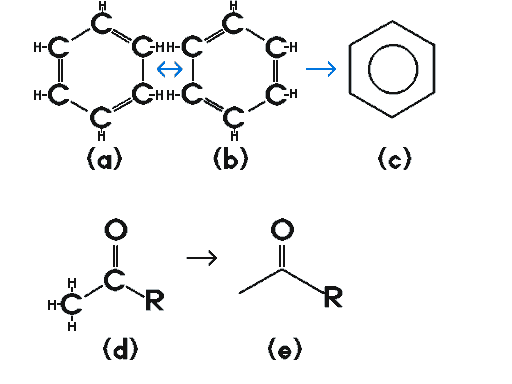 |
| Carbons which are double-bonded to an oxygen are called a "carbonyl" group. Thus, (d) has a carbonyl group which has a carbon on one side and an "R" group on the other side. "R" is used as a generic representation of an organic molecule or simply an atom, to enable chemists to make generalizations about families of chemicals which have certain parts of their chemical structure in common, except for the "R" group. (e) is the simplified version of (d). |
