The Nitrogen Cycle -
Control Ammonia and Nitrite
in Ponds, Lakes, Lagoons, Rivers
and Wastewater Treatment
by Valerie Anne
Edwards, CEO Alken-Murray Corporation
Ammonia forms when urease
enzymes produced by strains such as Escherichia coli, Hafnia elei Moller,
Ureaplasma urealyticum Shephard, Sporosarcina pastureii (Miguel)
Chester, (formerly Bacillus pasteurii), some Bacillus amyloliquefaciens
and other strains, when they contact urea and uric acid from animal urine
and water. Ammonia is also produced when proteins are degraded first to
amino acids and then to ammonia.
Special strains of Nitrobacter
winogradskyi and Nitrosomonas europaea, with superior talent
for rapid nitrification under aerobic conditions, are included in our Alken
Clear-Flo® 1100-50x, 1200, 1400-50x
, and 7110-50x,
formulas. Since molecular oxygen is involved in the reaction of
these strains, they are termed obligately aerobic. These autotrophic microbes
inefficiently use the energy gained from oxidizing ammonia to fix carbon.
This activity gives these bacteria a dual ecological role - recycling nitrogen
and fixing carbon into organic compounds. Carbon fixation by this method
is not very efficient, because the fixation of one mole of carbon requires
the oxidation of 35 moles of ammonia to nitrite and of 100 moles of nitrite
to nitrate.
Nitrifiers are fragile microorganisms
which are sensitive to acid despite the fact that they produce acid during
oxidation of ammonia and nitrite. If
a large source of nitrogen is dumped into the environment, these organisms
can potentially kill themselves by metabolizing it to nitric acid, unless
pH is buffered with limestone or other slow-dissolving sources of alkalinity.. Since they are also strict aerobes, nitrifiers
can be killed if the introduction of wastes leads to excessive growth of
other species that deplete oxygen. This is why Alken-Murray always recommends
mechanical aeration with two
to three ppm of oxygen when degradation of ammonia is desired.
Every year, 300 billion Kg, less
than one millionth of the total available nitrogen is recycled biologically.
Nitrogen released in an uncontrolled manner can have adverse environmental
impacts, such as elevated levels of nitrate in food and water, which may
constitute a health hazard to both humans and animals. Excessive N applied
to the soil then produces an accumulation of nitrate in plants, and undesirably
high levels of nitrate in potable water. In polluted waterways, excess organic
wastes stimulate bacterial growth, which consumes oxygen. Since artificial
aeration is not added to supplement oxygen levels, the dissolved oxygen
in the water becomes depleted, causing the fish to die, followed by death
of the lower forms, including protozoa. When oxygen completely disappears,
the water becomes septic, acquires a black color and supports only anaerobic
bacteria, which produce odors and toxic gases. By treating waste in lagoons,
waste pits and digesters, one of the point sources for excess nutrient runoff,
the responsible animal producer can reduce or eliminate these negative impacts.
Ideal management practices encourage the streams and lakes to slowly recover,
as various good anaerobic bacteria release oxygen during their metabolism,
which increases the dissolved oxygen level in the waterbody, until it can
again support normal aquatic inhabitants.
The biochemical reaction of
Nitrosomonas spp is:

The next stage is a direct
oxidation step, as follows:
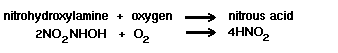
Sixty-six kilocalories of energy
are liberated per gram atom of ammonia oxidized.
The biochemical reaction of Nitrobacter
is a very simple reaction, involving the cytochrome system as follows:
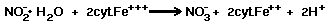
Then the cyt.Fe+++ is regenerated
by:

Eighteen kcal of energy is liberated
per gram atom of nitrite oxidized.
This whole process removes electrons
from a hydrated nitrite ion. The reactions of Nitrobacter are inhibited
by small quantities of ammonia gas (NH3: 1.4 mg/L inhibits 99%), which can
lead to a toxic buildup of nitrite, since Nitrosomonas is not inhibited
from oxidizing ammonia to nitrite, in the presence of ammonia.
Both Nitrosomonas and Nitrobacter
perform within a pH range of 6.8 to 8.5. Optimal pH is 8.2 to 8.3. Warmer
temperatures (above 60°F) also enhance nitrification. The size and type
of system, and degree of ammonia present, all influence the prescription
dosage and application site. Normally, treatment once or twice weekly with
Alken Clear-Flo 1100-50x or Alken Clear-Flo 7110-50x
is sufficient, after an adequate biomass is established. Higher
initial dosages are usually prescribed to establish a stable biomass rapidly.
Although there are a number of
different strains which will perform nitrification, the rate of formation
for Nitrosomonas is typically 1000 to 30,000 mgN/day/g dry weight
cells and for Nitrobacter is 5000 to 70000 mgN/day/g dry weight cells,
which is so much higher than the formation rates of the other strains capable
of nitrification, that these two are the most useful strains. Other bacterial
strains perform nitrification by forming hydroxylamine, amine oxides (R3N-O)
or nitroso- compounds (-N-NO or -NOH-NO containing compounds).
Recently, it has been discovered
that certain sulfide oxidizing, denitrifying bacteria, such as Paracoccus
pantotrophus and our own Bacillus mojavensi AMH 118, are also
capable of heterotrophic ammonia oxidation to nitrite. Both of these species
can be found in Alken Enz-Odor 6 and Alken Clear-Flo 1005. These formulas do NOT
replace true nitrifiers, but can augment performance in applications which
prohibit the use of true nitrifiers, due to lower oxygen level, acidic pH
range or levels of BOD and COD above 200 mg/L. Another product usefule when
conditions prohibit the use of true nitrifiers is Alken Nu-Bind 3, which utilizes Yucca schidigera
to inhibit the enzyme urease from turning urine into ammonia, while it provides
Bacillus mojavensis AMH 118 (mentioned above) and other Bacillus
spp. to utilize ammonia via both heterotrophic and chemotrophic pathways,
depending on the level of BOD, COD, ammonia and dissolved oxygen present.
Alken Nu-Bind 3 is often applied together with various
saprophytic heterotrophic microbial formulas designed to sufficiently degrade
BOD, COD and specific pollutants that dissolved oxygen levels rise to a
level where nitrifiers can survive to eliminate excessive ammonia that was
not needed by the heterotrophs to balance high levels of organic carbon
pollutants. The treatments applied in lakes, ponds, rivers and streams will
usually be different from those selected for industrial and municipal wastewater,
although some rivers in China and the Philippines are more polluted than
the average USA or EU wastewater treatment system and must be treated accordingly.
Denitrification is the reduction of nitrate to nitrite
and then to nitrous oxide or nitrogen gas. Denitrification is normally performed
under anoxic conditions, which is comparable to anaerobic conditions except
for the presence of nitrate and/or nitrite. In natural or aquaculture ponds
and lakes, this condition is found only in the sludge, but in wastewater,
the condition can be created by adding nitrate to collection systems, anaerobic
lagoons etc., or is created by addition of wastewater that formerly contained
ammonia, following nitrification, as described above. While Alken-Murray
offers a number of products with classical denitrification talent, only
Alken
Clear-Flo 1005 offers aerobic denitrification, performed
by Paracoccus denitrificans and two unique Bacillus pumilus
strains discovered by Alken-Murray's Valerie Anne Edwards. For each 1 mg/L
of nitrate reduced to nitrogen gas, you will recover 3.5 mg/L of alkalinity,
which is a less than perfect offset to the alkalinity needed to buffer acid
produced during nitrification.
Never apply nitrifiers CF 1100-50x/7110-50x
together with Alken Clear-Flo 1005
since the faster growing aerobic denitrifiers in the latter product, will
competitively exclude Nitrobacter winogradskyi in the nitrifier product,
when both compete for nitrite, under low to moderately aerobic conditions.
This is not an issue with classical denitrifying bacteria, found in such
products as Alken Clear-Flo 1006, 7007, 7008 etc., since classical
denitrifiers prefer free oxygen to nitrate and will use nitrite ONLY when
no free oxygen or nitrate are available instead
Diagram of the Nitrogen Cycle
Great new scientific
paper examines genes of Nitrosomonas europaea |
